What Is Waardenburg Syndrome?
Waardenburg syndrome is a condition named after Dutch ophthalmologist Petrus Johannes Waardenburg. In 1947, he first described a patient with signs and symptoms of Waardenburg syndrome. In 1951, after the identification of several other individuals with similar symptoms, he defined it as Waardenburg syndrome type 1.
Eventually, it was found to have four different types. Now, some types are further subdivided into more subtypes based on the gene that is responsible for the condition. Waardenburg syndrome accounts for 2% to 5% of individuals with congenital hearing loss.
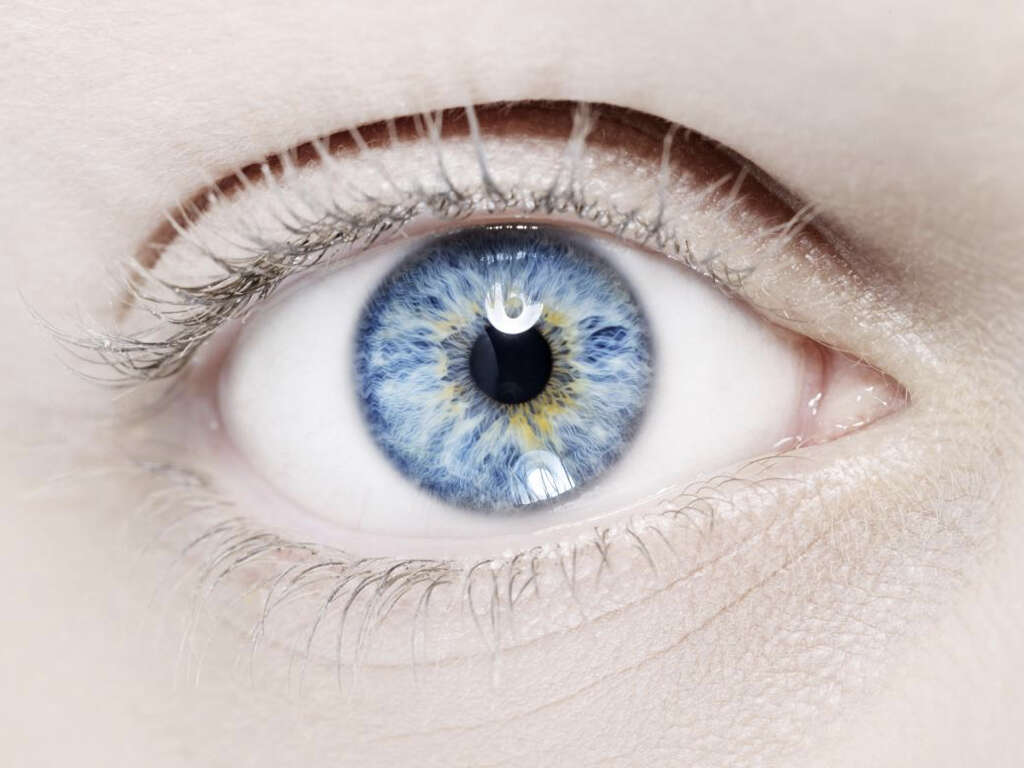
1. Signs and Symptoms
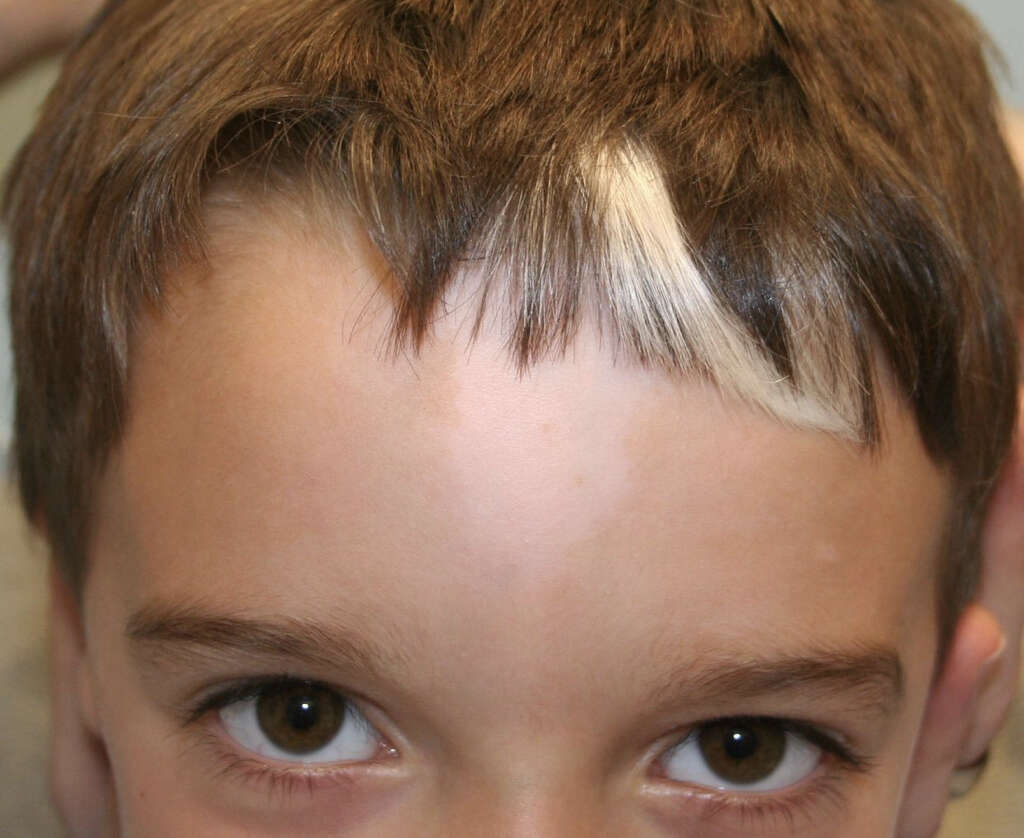
The signs and symptoms of Waardenburg syndrome differ based on the type of syndrome. Some of the symptoms include brilliantly blue or pale eyes or different colored eyes such as in complete or sectoral heterochromia. Affected individuals can also have premature graying or a forelock of white hair known as poliosis, wide-set eyes, broad nasal root, moderate to profound hearing loss, white skin pigmentation, cleft lip, neurologic manifestations, and a low hairline with eyebrows that meet in the middle. individuals with Waardenburg syndrome have also been reported to have congenital defects such as spinal defects, intestinal defects, and elevation of the scapula. Cognitive abilities are not generally affected.

2. Gene Mutations
In Waardenburg syndrome, there are mutations in various genes to cause different types of the syndrome. There are 6 genes that are involved in this condition: PAX3 gene that encodes the paired box 3 transcription factor, EDN# gene (endothelin 3), MITF gene (microphthalmia associated transcription factor), SNAI2 (snail homolog 2), SOX10 (encodes the Sry bOX10 transcription factor), and EDNRB (endothelin receptor type B).
Waardenburg syndrome generally has an autosomal dominant pattern where only one copy of the abnormal gene is required to cause disease. This can be seen in types 1 and 3 while types 2 and 4 have an autosomal recessive type of inheritance pattern. A small portion of cases are due to new genetic mutations.
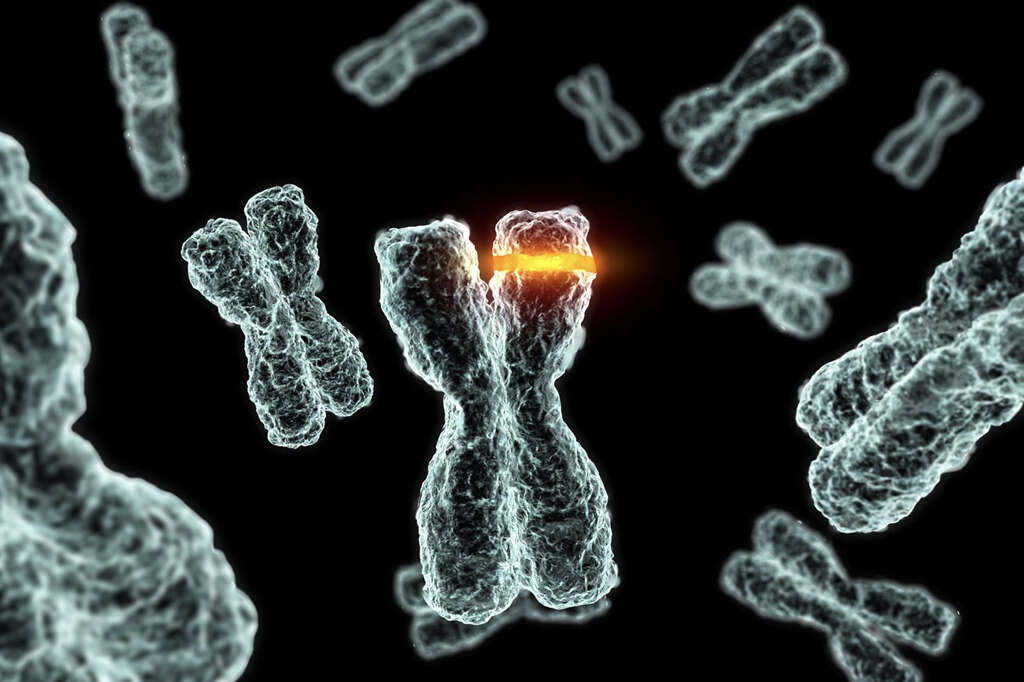
gene (endothelin 3), MITF gene (microphthalmia associated transcription factor), SNAI2 (snail homolog 2), SOX10 (encodes the Sry bOX10 transcription factor), and EDNRB (endothelin receptor type B). Waardenburg syndrome generally has an autosomal dominant pattern where only one copy of the abnormal gene is required to cause disease. This can be seen in types 1 and 3 while types 2 and 4 have an autosomal recessive type of inheritance pattern. A small portion of cases are due to new genetic mutations.
In 1916, Dutch ophthalmologist Jan van der Hoeve first reported symptoms of Waardenburg syndrome in two deaf twin girls. in 1947, As previously mentioned, Waardenburg syndrome was described by Dutch ophthalmologist Petrus Johannes Waardenburg. It has a less popular name of Waardenburg-Klein syndrome.
This is because it was also described by David Klein, a Swiss geneticist and ophthalmologist. In 1948, Klein and Waardenburg collaborated and performed a search among 1,050 deaf patients. In 1971, Arias drew attention to several patients as a separate subtype of the syndrome (type 2), which were overlooked by Waardenburg. In 1981, Shah and colleagues reported 12 infants with Waardenburg syndrome associated with Hirschsprung disease.

3. History
Waardenburg syndrome type 1 is believed to be caused by mutations in the PAX3 gene found on chromosome band 2q35. Reports have included splice site, nonsense mutations, frame shifts, whole gene deletions, and more. In this subtype of Waardenburg syndrome, it can be inherited via an autosomal dominant pattern or due to a spontaneous mutation.
In some Chinese patients with type 1, two nonsense PAX3 mutations were identified where one is heterozygous for a previously reported mutation among the European population (R223X) while another is heterozygous for a novel nonsense mutation (S209X). Both of these mutations have led to the discontinuation of the PAX3 protein. SOX10, MITF, and PAX3 mutations have been reported in Chinese patients. Type 1 patients may have congenital hearing loss and almost always have widely spaced eyes.

4. Waardenburg Syndrome Type 1
Some cases of Waardenburg syndrome type 2 have been reported to be due to a mutation in the MITF gene found on chromosome band 3p14.1-p12.3. Like Waardenburg syndrome type 1, it can be inherited via the autosomal dominant pattern or due to spontaneous mutation.
There are also other cases of Waardenburg syndrome type 2 that are linked to mutations on 1p21-p13.3 while many other cases are unlinked to other unknown mutations. Type 2 Waardenburg syndrome can be further divided into 4 other subtypes. Patients with this type often have heterochromia of the irises (different colored eyes) and permanent hearing loss.

5. Waardenburg Syndrome Type 2
In patients with Waardenburg syndrome type 3, mutations in PAX3 have also been found. It is thought that it may be inherited in an autosomal dominant pattern. There are some cases where it is thought to be due to a manifestation of homozygous mutations of the gene.
In type 3, the patients may experience changes in their skin pigmentation, progressive hearing loss, and anomalies of the upper limbs due to minor defects in structures arising from the neural crest. There can be fusion of the carpal bones, hypoplasia of the musculoskeletal system, and flexion contractures. Waardenburg syndrome type 3 is also known as the Waardenburg-Klein syndrome. It is the rarest form of Waardenburg syndrome.
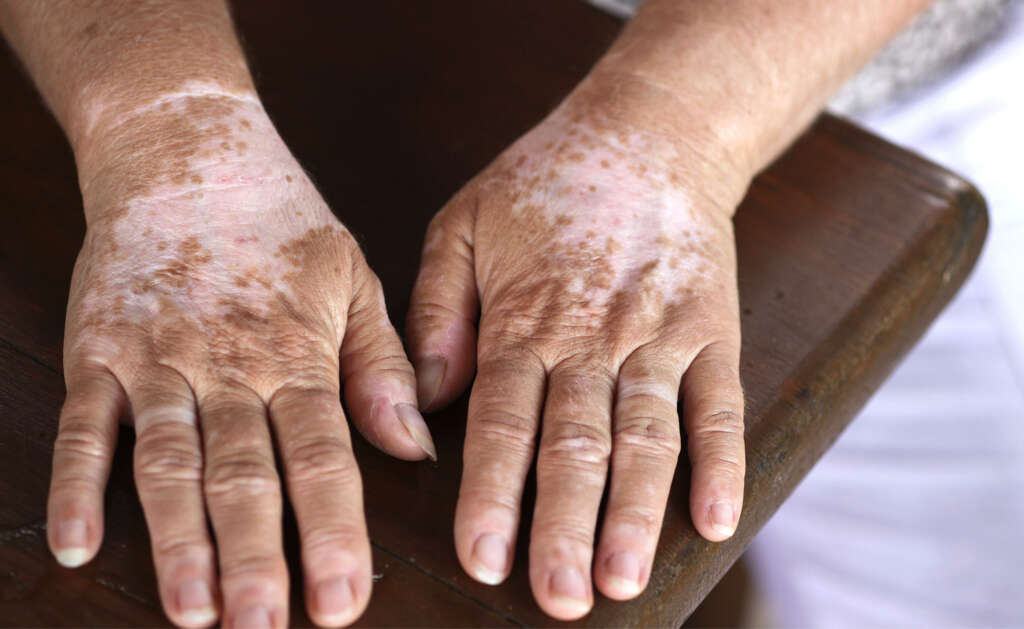
6. Waardenburg Syndrome Type 3
Waardenburg syndrome type 4 has been studied using a zebrafish model and is thought to be caused by homozygous mutations in the EDNRB or EDN3 gene. These mutations lead to Hirschsprung disease. Other mutations that have been reported in this subtype include the SOX10 gene.
When SOX10 mutations are present, it is thought to cause a more severe type where there is central dysmyelinating leukodystrophy and peripheral demyelinating neuropathy. It can be further divided into 3 other types. Also known as the Waardenburg-Shah syndrome, patients tend to experience hearing loss, pigmentation in the hair, skin, or eyes, and Hirschsprung disease resulting in severe constipation and intestinal blockage.
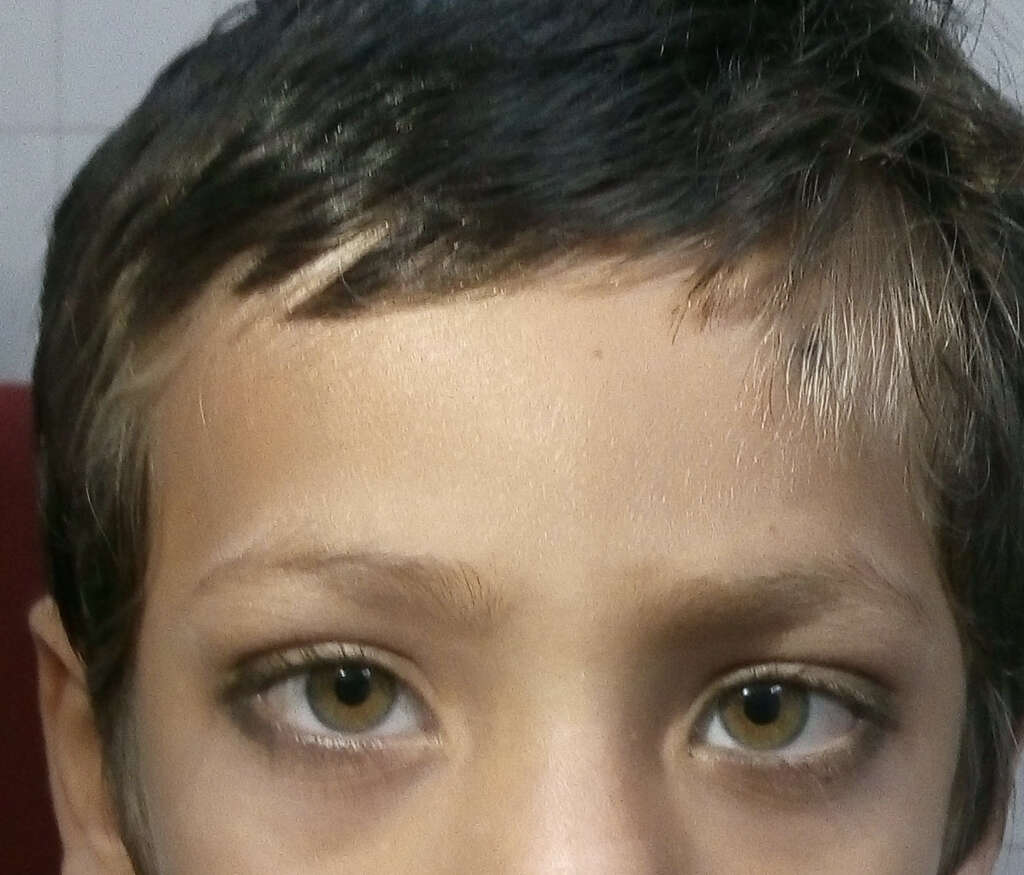
7. Waardenburg Syndrome Type 4
Since there are no cures for Waardenburg syndrome, the aim of treatment and management for this condition is supportive. This means that it is aimed at reducing the number of complications and increasing the quality of life for patients with Waardenburg syndrome. For example, since the most likely symptom to be of practical importance in this condition is deafness, it can be treated as any other irreversible deafness.
Children born with congenital deafness will need extra support as deafness can impede their learning process compared to their peers. In some cases where there are cosmetic issues, they may benefit from consultations with experts in the field. Other abnormalities such as structural, neurological, and Hirschsprung disease can also be treated based on their symptoms.

8. Treatment and Management
Since it is estimated to affect 2% to 5% of those with congenital hearing loss, this means there is an estimated prevalence of 1 in every 42,000 individuals. However, it is estimated that the actual prevalence may be as high as 1.19 to 2.08 per 10,000 people. Types 1 and 2 have been observed to be the commonest type of syndrome while types 3 and 4 are considered rare. As of 2002, there are only 48 reported cases of type 4 Waardenburg syndrome. In schools for the deaf, it is estimated that Waardenburg syndrome affects 1 in 30 students.
It affects all regardless of gender or race. Since there can be a highly variable presentation, it can be difficult to estimate the precise figures of the condition’s prevalence.
9. Epidemiology
Since Waardenburg syndrome is a genetic condition, there is no cure. It is also rare and support groups can become important to help connect patients with other patients and families to help provide additional support. There are also organizations that provide patient centered information that can be crucial to increase awareness and educate patients.
These support and advocacy groups are vital as they can help direct patients and family members to other resources, research, clinical trials, and services. There are also organizations that have their own experts who are available as medical advisors or are able to provide lists of clinics and doctors. Online support groups and forums can also be beneficial for moral support.












