What Is an Ionic Bond?
2. Ionic Bonding
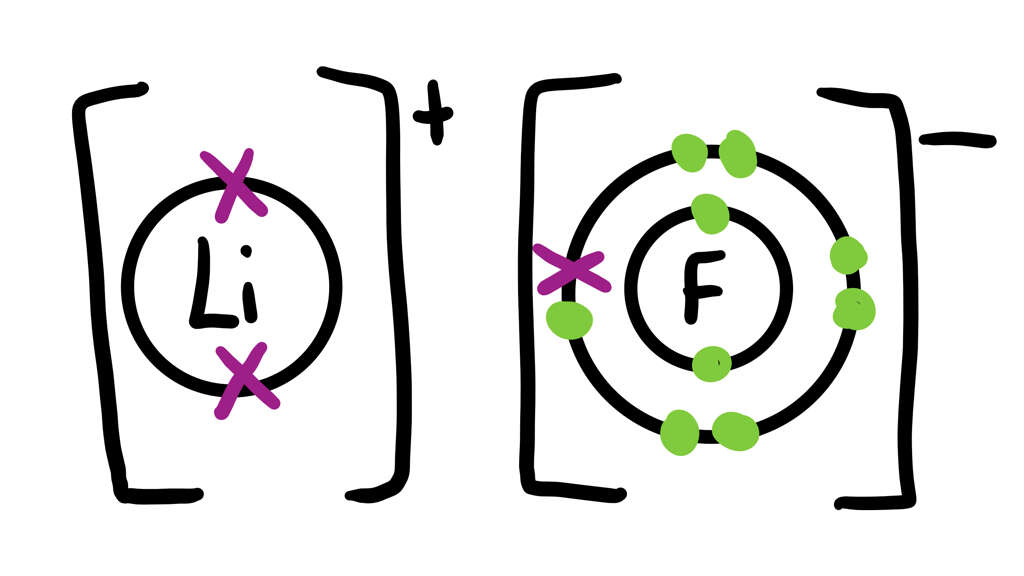
You are able to tell which charge an ion will usually have by their position on the periodic table. Atoms with a negative or positive charge will be attracted to atoms that have the opposite charge to themselves. Ionic bonds are formed between metals and non-metals. The bond forms when the non-metal acquires one of the metals “spare” electrons.
Advertisement
Both atoms are now bonded by an electrostatic attraction; a bond is formed between the two as the electron holds them together tightly. When this bond takes place, the non-metal becomes a negative anion and the metal becomes a positive cation.
Advertisement
Advertisement





