What Is an Ionic Bond?
Our whole world is made up of chemicals, including gases, solids, and even the water that we drink. There are numerous different types of chemicals and each one consists of different combinations of atoms. Water, for example, is always made from two hydrogen atoms and one oxygen atom.
Different types of chemicals are made from atoms that are bonded to each other in different ways. One such method is ionic bonding, and you will see examples of ionic bonding in your own home. Here’s a close look at what ionic bonding is and some examples of where we see it in action.

1. Ions
In order to answer this question, it first helps to understand what an ion is. In short, an ion is a molecule or atom that has a net electrical charge. Atoms are made up of protons, neutrons, and electrons. A neutron has no electrical charge, a proton has a positive electrical charge, and an electron has a negative electrical charge. Many atoms will have no charge because the numbers of protons and electrons cancel each other out. An ion, however, will not have the same number of protons as electrons, and this leaves the atom with a positive or negative charge overall.
2. Ionic Bonding
You are able to tell which charge an ion will usually have by their position on the periodic table. Atoms with a negative or positive charge will be attracted to atoms that have the opposite charge to themselves. Ionic bonds are formed between metals and non-metals. The bond forms when the non-metal acquires one of the metals “spare” electrons.
Both atoms are now bonded by an electrostatic attraction; a bond is formed between the two as the electron holds them together tightly. When this bond takes place, the non-metal becomes a negative anion and the metal becomes a positive cation.
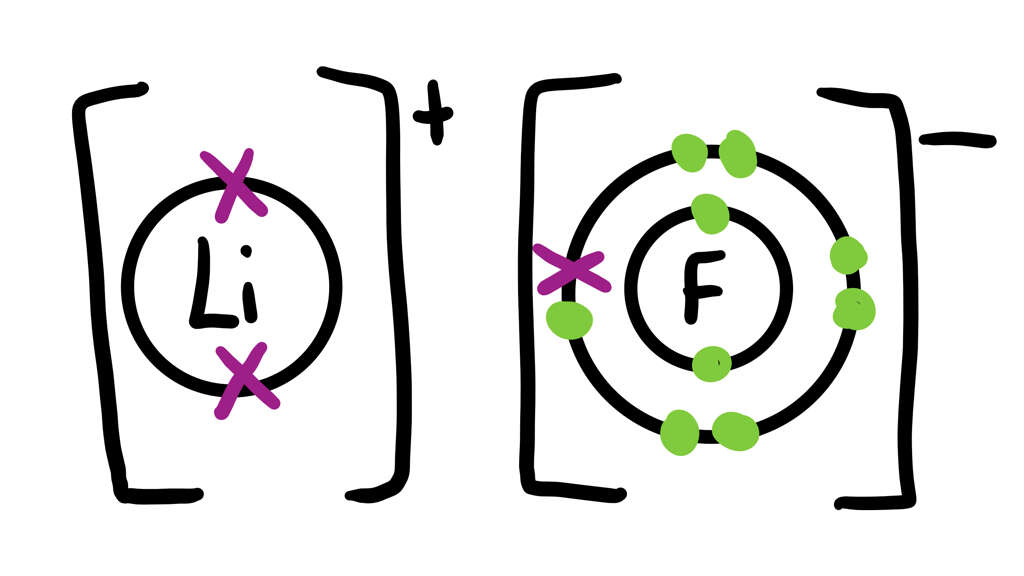
3. Salt
There are countless examples of ionic bonds all around us, you probably even use one at the dinner table. Atoms of certain elements will form bonds with elements from other atoms; causing the two to combine to create something else. One of the most common examples you will encounter is salt.
Salt is made when sodium (Na) oxidizes and loses an electron, causing it to become positively charged (Na+). The chlorine atom (Cl) will gain this electron, causing it to become negatively charged (Cl-). They are now two electrons with opposite charges, and this causes the two to become attracted to each other.

4. Ionic Lattice
Different chemicals are combined from different elements in ways that determine their overall structure. For example, water (H2O) has a loose structure with few bonds between atoms and this results in liquid. In cases of ionic bonding, the atoms have more connections between other atoms and this helps to make a rigid structure.
This structure is known as an ionic lattice. In the case of salt, each sodium ion will be attracting all nearby chlorine atoms, and all chlorine atoms will be attracting all sodium atoms. This results in all of the atoms being packed tightly in together, resulting in the lattice structure.

5. Soluble in Water
Drop some salt in water, and it will quickly become dissolved and it will continue to dissolve until the water is saturated with the chemical. The same also happens in other chemicals that have been formed by ionic bonds. Water is a dipole, this means that it has a pair of oppositely and equally charged poles. When you put salt, for example, into water, the water molecules attract the negative and positive ions.
This then causes the sodium and chlorine atoms to break away from each other. The separated particles are then able to move around within the solution separately from each other.

6. Conductivity
Salt does not conduct electricity, and nor do other chemicals that have been formed by ionic bonds. This is because their ions are fixed in place and not free to move from place to place; something that is necessary for electricity to be able to pass through a substance. Salt and other ionic compounds can form conductive solutions when dissolved in water, however.
This is because when dissolved, the ions are now free to move, allowing electrical charges to pass through. Of course, water does not need ionic compounds to be dissolved in it for it to be conductive, because its own ions are also free to move.

7. Covalent Bonds
While salt and many other chemicals are formed using ionic bonds, many other chemicals are formed using covalent bonds. As mentioned, an ionic bond is formed because two atoms of opposite charges are attracted to each other. Covalent bonds, however, are formed when two atoms share one or more of the same properties.
This type of bonding occurs because the nuclei of both atoms will both have an attraction for the same electrons. In some instance, a single atom, such as a hydrogen atom, will share an electron with more than one other atom, such as oxygen, to make the well-known chemical we know as water.
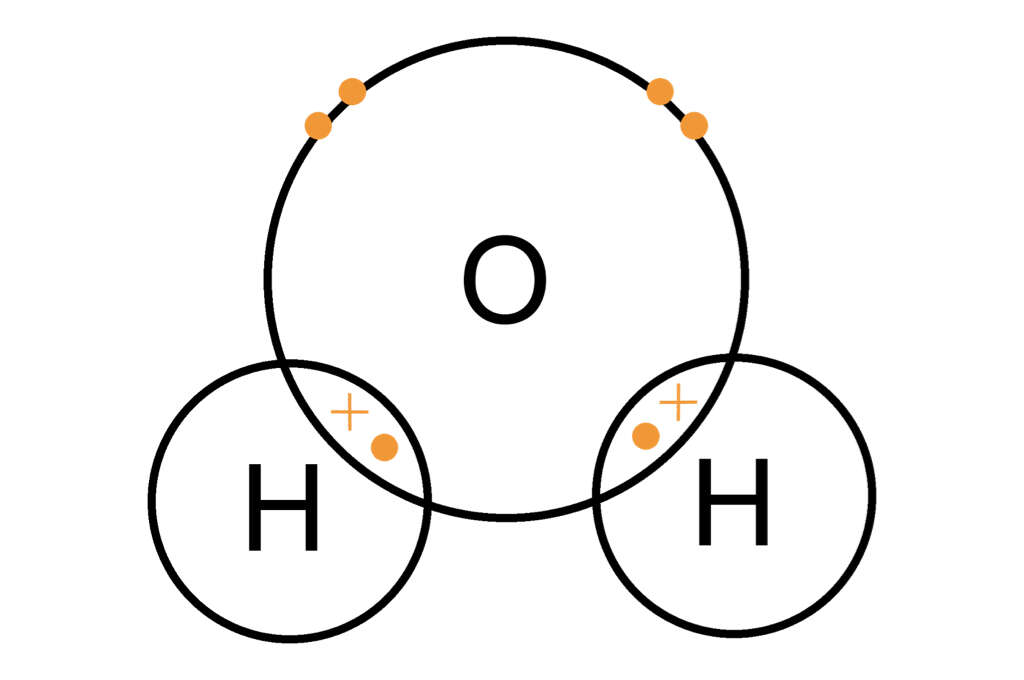
8. Ionic Bonds Tend to Be Solids
Examples of chemicals made through covalent bonds include methane, carbon monoxide, and water. Covalent bonds will generally form gases and liquids because the nature of the bond forms complete molecules. In ionic bonding, however, molecules continue to attract other nearby molecules, forming the lattice structure that means ionic compounds are generally solids.
There are some examples of liquid ionic compounds and these are generally known as liquid electrolytes, liquid sales, ionic glasses, and/or ionic melts. These generally refer to ions that are liquid at temperatures below the boiling point of water (100 C). Ionic liquids can also become solids when they are cooled sufficiently.

9. Ionic Bonds Are Stronger
Ionic bonds are generally a lot stronger than covalent bonds. This is actually quite easy to demonstrate because it is so easy to separate water just by pouring half a cup into the other. Similarly, gases can be separated simply by closing the lid of a jar, leaving the gas inside separated from the gas outside.
In the example of salt, however, separating grains of salt is nowhere near as easy. It can be done, of course, but it takes a great deal more energy than separating gas or water. This is down to the strength of the attraction between ions of opposite charges.

10. Boiling Point
It is well known that water, a chemical formed by covalent bonds, has a boiling point of (100 C) at sea level. The temperature at which water will boil will decrease according to altitude because the air pressure is lower the higher up we go. The boiling point of water is often used as a benchmark, and it boils at a lower temperature than ionic compounds do.
The strength of the bond in ionic compounds is stronger than those in covalent compounds. This means that more energy is required to break these compounds, and this means more heat, resulting in a higher boiling point.